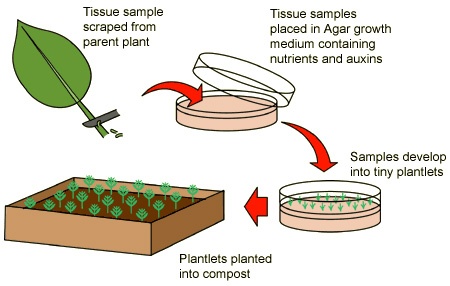
Micropropagation, or tissue culture as it is also called, is the most cutting-edge means of propagating plants. It involves propagating plants from small plant parts, tissues or cells in specialized conditions in which the growing environment and nutrition are strictly controlled.

Click here to view a video that explains how to make plant tissue cultures.
The basic principles of tissue culture have been known for about 100 years and such theories were suggested as long ago as the early 1800s. By 1939 scientists in the United States and France had made significant discoveries. Within another ten years, researchers had laid a solid foundation for today’s large-scale tissue culture laboratories, propagation facilities, and further advances through ongoing research. It was not until recent decades that micropropagation became a feasible means of producing plants for the nursery industry. Today, many plants are propagated in this way. In catalogues, the names of plants that have been propagated by tissue culture methods are often followed by “TC” in parentheses. With every passing year more and more, advances are made, and an increasingly wide range of plants find their way into the many tissue culture facilities which are appearing at an equally rapid pace.
Tissue culture makes use of an in vitro system. In vitro is from the Latin for “in glass,” which is in reference to the fact that plant tissues are developed in test tubes and flasks under laboratory conditions. The multiplication of plants in vitro does not create a new process within the plant, it simply directs and enhances the plants natural potential to put forth new growth and multiply in a highly efficient and predictable way.
There are several advantages to micropropagation when compared with traditional asexual methods of propagation.
- Plants can be mass-produced rapidly.
- A new plant can generally be introduced to the nursery industry more quickly.
- Tissue cultured plants are free of insect and disease pests when removed from test tubes.
- The growth of in vitro cultures requires little care on a day to day basis, apart from casual surveillance.
There are also several disadvantages.
- More expensive. The start-up costs for a commercial micropropagation facility are high.
- The techniques used require greater skill and training.
- Not all plants can be produced through tissue culture.
- Mutations may occur during the culturing process resulting in plants being different from the parent. This can be disastrous if not noticed at an early stage.
The success of tissue culture for reproducing new plants is based on the ability of small plant parts, tissues or cells to undergo rapid cell multiplication under the proper chemical and physical conditions, and then to differentiate into the various parts that make up an entire plant. The plant part, tissue or cell type that is removed from a stock plant for purposes of being cultured is known as the explant. The explant can be a shoot tip, root tip, leaf tissue, pollen grain, seedling tissue, bulb scales and others.
There exist a number of factors that will affect the success of generating new plants by micropropagation. Sterility is of the utmost importance at all stages. Lab conditions are essential and much of the great expense is attributable to the need for such facilities. All surfaces with which the explant may come in contact, including countertops, tools and human hands, need to be sterile. The growth medium and glassware in which the new plants will be cultured must also be sterile. Apart from sterility, the explant itself and the culture conditions (light, medium, temperature) all play significant roles in the success.
The type of explant taken from a parent plant will also affect the generation of new cell growth and the subsequent new plants. Certain explant types work better for certain plants.
The medium used in tissue culture is unlike that used in any other type of plant propagation. A semi-solid, gelatinous material called agar is used. This provides support for the culture, but by itself is essentially inert. What is mixed in with the agar is what stimulates new growth. These ingredients will vary depending on the plant being cultured, as will the concentrations used. Ingredients include inorganic salts of many essential plant nutrients and organic compounds like carbohydrates, vitamins, various hormones and growth regulators.

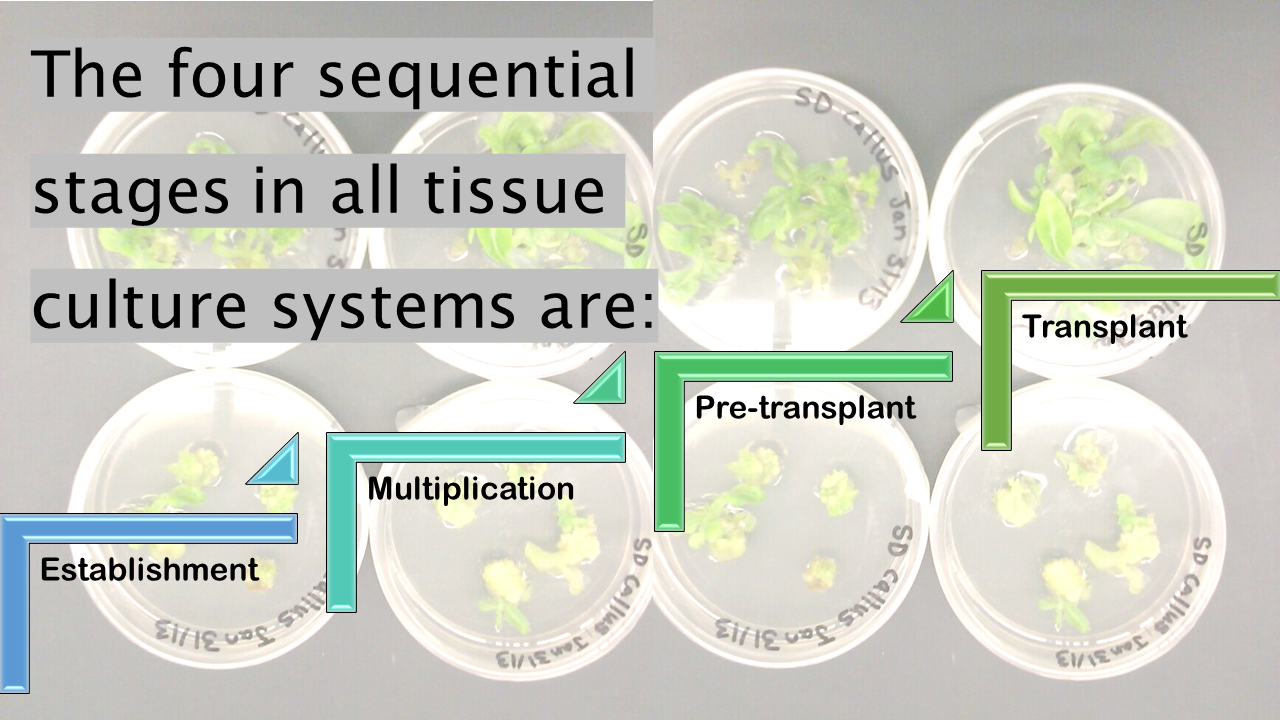
The purpose of the establishment stage is to establish a sterile explant in culture. The initial explants from the first stage have developed a mass of shoots that are separated into individual propagules and transferred to a fresh medium culture. This second medium is frequently the same or similar to that used in Stage 1, but the concentrations of certain ingredients may be altered.
The pre-transplant stage is necessary to prepare the grown propagules, now known as plantlets, for the shift from the rigidly controlled in vitro environment to that of a more typical plant growth environment, usually in a greenhouse. The pre-transplant stage also offers an opportunity to cull mutated propagules or ones that somehow became infected with a disease.
The transplant stage is the point at which the plantlets are moved to a pasteurized soil mix essentially as seedlings would be transplanted. At this point the plantlets are very tender and dry air and/or bright sunlight can easily burn them. Gradually, humidity levels can be reduced and more sunlight provided to the young plants at which point they should be established and growing under standard conditions.
Propagation Tools
The following tools are used in the propagation methods described above.
Budding Knife – A razor-sharp knife used to make cuts on the seedlings and to cut off the bud-eye. The knife must always be sharp and in good working condition to prevent tissue damage to the plant when cutting through it. If tissue damage occurs, the graft will most likely fail.
Budding Tape – Clear polyethene strips, used to maximize contact between the bud and the rootstock until the union and the healing is complete. It also prevents drying and excess water from getting in and rotting the bud.
Pruning Shears – Bud-wood is cut using pruning shears. Pruning shears are also used where cuttings are used for propagation.
Sharpening Stone – All blades become blunt with use and require periodic sharpening. A sharpening stone, or wet stone, and honing oil are required.
Sterilization Liquid – Knives and shears must be periodically cleaned and sterilized properly with a solution of 10% bleach (Jik).