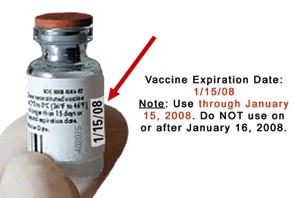
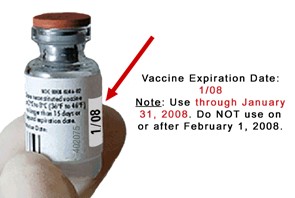
All vaccines and diluents have expiration dates. The expiration date is the date by which the vaccine or diluent should be used. This date is printed on all vaccine and diluent vials and boxes. Expiration dates vary by the type of vaccine or diluent, and by the lot number. The vaccine or diluent may be used up to and including this date unless otherwise stated in the product package insert. Vaccine and diluent should not be used after this date has passed. When the expiration date is marked with only a month and year, the vaccine or diluent may be used up to and including the last day of the month indicated on the vial. Any unused vaccine or diluent should not be used after this month has passed.
 |
 |
Expired vaccines and diluents, even if they are only 1 day past the expiration date, should never be administered. Likewise, vaccines that have been mishandled and lost their potency because of inappropriate storage conditions should not be administered. If a dose of expired or mishandled vaccine is given by mistake, the dose should not be counted as valid and should be repeated, unless serologic testing indicates that an adequate response to the vaccine has been achieved. Promptly remove expired or mishandled vaccines and diluent from the refrigerator or freezer and dispose of it appropriately. Contact the vaccine supplier, which may be the vaccine manufacturer for specific policies regarding the disposition of mishandled or expired vaccines.
The expiration date printed on each vial or box assumes the vaccine has been properly transported and stored at all times and that it has not become contaminated. If the vaccine has been inappropriately exposed to excessive heat, cold, or light, its potency may be reduced before the expiration date is reached. The only way to determine if proper transport and storage conditions have been maintained is to monitor vaccine and diluent temperatures during every link in the cold chain and to safeguard HPV, MMR, MMRV, rotavirus, varicella, and zoster vaccines from exposure to light. The expiration date printed on each vial or box may also be invalidated after the vial is opened or reconstituted.
Multidose premixed vaccine vials contain bacteriostatic agents that prevent the growth of bacteria. These vaccines can be used until the date of expiration printed on the vial unless they become contaminated.
Single-dose vials are meant for one-time use only. Once the protective caps on single-dose vials have been unsealed, it may not be possible to determine if the rubber seals have been punctured. Therefore, do not open single-dose vials until you are ready to use them. To avoid needless waste of vaccine, always check the vial before removing the cap to make sure you have the correct vaccine type, and remove the cap only when you are ready to draw up and administer the vaccine. Single-dose vials without their protective caps should be discarded at the end of the clinic day.
The vaccine coordinator should ensure that someone rearranges the placement of vaccine and diluent supplies according to the expiration dates on a weekly basis and each time a vaccine shipment arrives. The vials and boxes with the earliest expiration dates should be placed in front of other vials and boxes of the same type with later expiration dates. This practice avoids waste by ensuring that vaccines and diluents with the shortest expiration dates are easily accessible and will be used first, thereby limiting the amount of unused vaccine that has passed their expiration date.
Expired vaccines and diluents should never be administered. Promptly remove expired vaccines from the refrigerator or freezer to avoid accidental use.