Completion requirements
View
GMP guidelines are not prescriptive instructions on how to manufacture products. It is a series of general principles that must be observed during manufacturing. When an organisation is setting up its quality program and manufacturing process, there may be many ways it can fulfil GMP requirements. It is the organisation's responsibility to determine the most effective and efficient quality process. Good manufacturing practices are largely governed by the Foodstuffs, Cosmetics and Disinfectants Act. (Act no 54 of 1972), 27 June 2003.
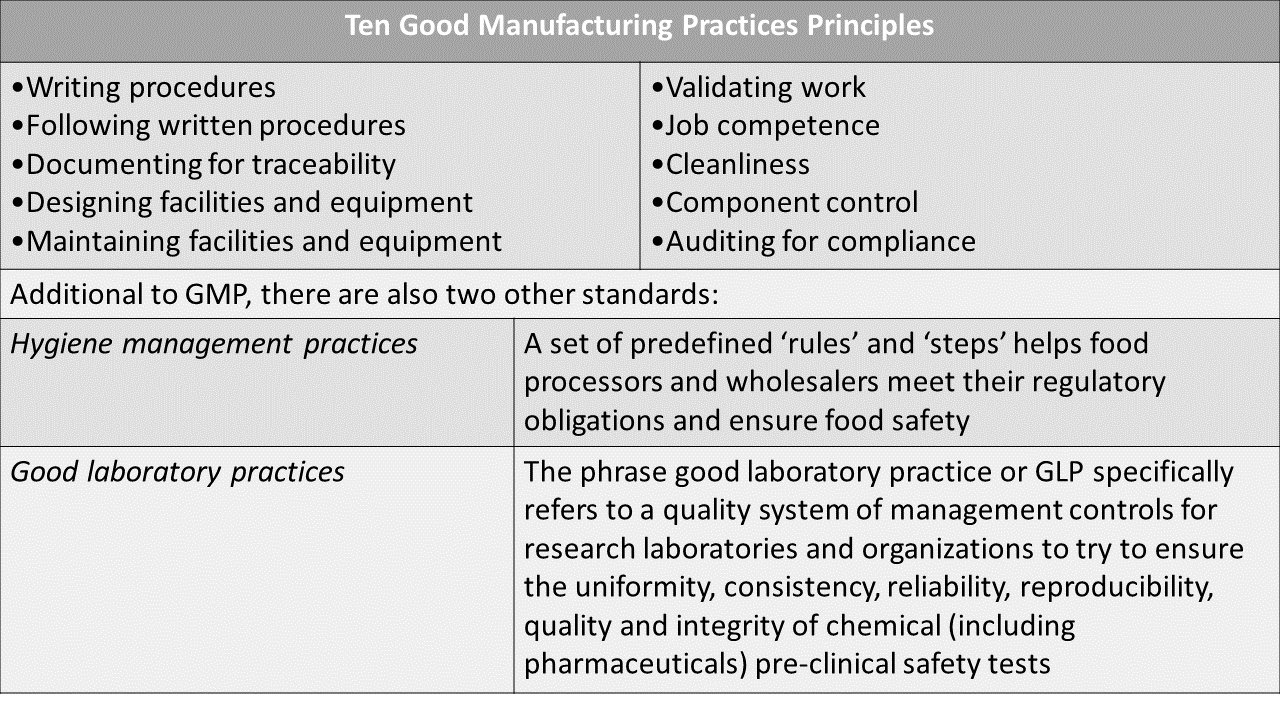
GMP contains ten principles that introduce employees to critical behaviours established by FDA and industry leaders to maintain good manufacturing practices in plants.